A study published yesterday in Cell[1] has purportedly found that memories formed as infants may be able
to be retrieved as adults using optogenetic techniques, and various media
outlets have enthusiastically inferred that this suggests we may be able to
remember the events from our infancy, some even suggesting we may be able to
remember our own birth.
The study, led by psychologist Paul Frankland, was based on
previous research by the group which found that the “forgetting” of memories during
infancy may be a result of high levels of hippocampal neurogenesis at this age –
i.e. the neurons representing the memories are replaced by new neurons, thus
erasing the memories.
The aim of the current study was to determine if the
memories formed during infancy are permanently lost due to a failure in
encoding during infancy, or become progressively inaccessible over time due to
a progressive loss in the ability to retrieve them as the mice age.
The researchers placed young mice into a box and gave them a foot-shock, so that when they are placed back into the box they freeze in anticipation of the shock - a classic fear-based training paradigm. The mice had been engineered to contain a specific set of light-sensitive neurons in a particular region of the hippocampus involved in the formation of memories - the dentate gyrus - which allowed the researchers to activate these neurons by firing a laser at them. They then placed the same mice back into the box as adults and activated this set of neurons, thus reinstating the memory and causing the mice to freeze in anticipation of the shock.
The researchers placed young mice into a box and gave them a foot-shock, so that when they are placed back into the box they freeze in anticipation of the shock - a classic fear-based training paradigm. The mice had been engineered to contain a specific set of light-sensitive neurons in a particular region of the hippocampus involved in the formation of memories - the dentate gyrus - which allowed the researchers to activate these neurons by firing a laser at them. They then placed the same mice back into the box as adults and activated this set of neurons, thus reinstating the memory and causing the mice to freeze in anticipation of the shock.
The authors seem to suggest that this means that some hidden
traces of the memories created during infancy were retained and were able to be
recalled by the researchers by optogenetically activating the specific set of hippocampal
neurons which were observed to be activated during the contextual fear encoding
(the “dentate gyrus encoding ensemble”), positing that the reactivation of
these ensembles was sufficient for “memory recovery” in adulthood.
They then quantified the activity of an activity-regulated
gene, c-Fos, in cortical and
subcortical brain regions following the fear learning event. The purpose of
this was to determine whether fluorescently-tagged neurons activated during the
memory encoding event were preferentially reactivated during the “memory recall”
in adulthood – which of course they were, implying successful recall of the
memory.
However, while they may have been able to reactivate a
neural pathway they have essentially programmed into the mice’s brains during infancy,
I’m not so sure it was the actual memories themselves which were “recovered”.
It’s worth nothing that these are memories which the
researchers have created in the mice by giving them foot-shocks within a
particular environmental context; they are not naturally formed memories.
“When the infant mice were placed in the box and the laser was turned on, the animals’ memories of the electric shock returned and they froze in place.”
The fact that the mice freeze when they are placed into the same
environment in which they were given a foot-shock during infancy does not
necessarily mean that they remember receiving the foot-shock. It simply means
that a particular behaviour (freezing) has been programmed into their brains
and artificially re-instated by firing lasers at the neurons underlying this behaviour.
It means that the same neural pathways resulting in a fear-based freezing response
were activated – but these may be totally separate from the pathways containing
the actual memory of the event (if they even exist). Besides, we are talking
about a simple, conditioned response here; an instinctual behaviour in response
to pain – much like you learn to quickly move your hand away from a hot plate –
not an actual subjective, detailed memory of an event. It’s possible that the
mice would freeze if placed in an entirely different context and the same light-sensitive
neurons artificially reactivated.
“To first induce memory formation in the animals, the scientists placed the mice in a box and gave them a mild foot shock. While young adult mice retained this memory and froze when put in the box a second time, infant mice forgot this fear-related memory after a day and behaved normally when they encountered the box again”
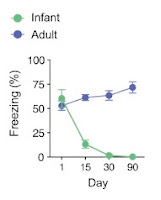 |
| Percent freezing levels declined with retention delay in P17, but not P60, mice. From Guskjolen et al., 2018, Figure 1(B). |
So the infant mice were not forming the memories? This appears to contradict
the conclusions of the study – i.e. that those memories are simply hard-to-retrieve;
hidden deep within the brain, unable to be recovered by natural cues, and that
direct stimulation of the engram (in combination with re-exposure to the training
context) may reinstate the connections, leading to memory recovery. How can we
say that the memories are simply difficult to retrieve when we are unsure if
they were ever formed in the first place?
“However, we found that opto-stimulation of neural ensembles that were engaged during training was sufficient to induce conditioned freezing at the same retention delays. These results suggest that the underlying engram corresponding to the fear conditioning event is not completely overwritten. Rather, this engram presumably exists in an otherwise inaccessible, dormant state, in which “natural” reminders (such as exposure to the training context) most often do not induce successful reactivation(...) This pattern of results is reminiscent of other amnestic states, including mouse models of retrograde amnesia and Alzheimer’s disease, in which opto-stimulation of tagged encoding ensembles (but not presentation of natural cues alone) permits memory recovery."
Again, the conclusions drawn assume that the artificially activated
ensembles encode the actual memory of the event itself – which is not only not confirmed,
but hard to believe considering when the mice were put back in the box the
second-time as infants, they had not remembered the fear-related memory
supposedly created the day before. How, then, do we know that the memory was
encoded at all? How do we know it is the memory that is recalled, and not
simply a programmed, artificially instated fear-response? This is too
simplistic of a model to draw such far-fetched conclusions, and we certainly can’t
say that this type of “forgetting” in infancy is akin to other types of amnesia
such as that in Alzheimer’s disease. They are completely different processes,
at completely different ages.
Furthermore, less cortical “re-engagement” was observed following
optogenetic stimulation of the dentate gyrus engrams in mice trained as infants
compared to those trained as adults, further highlighting the possibility that
the memories which were purportedly retrieved may not have been formed at all
in the infants.
“Indeed, whereas adult contextual fear memories are successfully consolidated over the course of weeks, equivalent infant memories are being actively forgotten during this period and therefore perhaps not successfully consolidated in the cortex (…) opto-stimulation of tagged dentate gyrus ensembles leads to recovery of an engram that is qualitatively different (and likely impoverished) compared to the equivalent representation in adult animals.”
The authors even concede that the “memory recovery” did not
persist into the light OFF periods – i.e. when the trained mice were placed
into the box as adults, they did not freeze unless the hippocampal engrams engineered to be light-sensitive were activated by the researchers – a pattern which has been observed in similar studies involving reactivation
of tagged engram cells in the dentate gyrus [2–7].
While the study further adds weight to the idea that infantile
forgetting is likely due to a failure of memory encoding in the infant brain (something
which we knew anyway), the methods used are simply insufficient to be able to
draw some of the conclusions the authors propose, and the study certainly does
not suggest that we may be able to recover our infantile memories anytime soon.
[1] A. Guskjolen, J.W. Kenney, J. de la
Parra, B.A. Yeung, S.A. Josselyn, P.W. Frankland, Recovery of “Lost” Infant
Memories in Mice, Current Biology. 0 (2018). doi:10.1016/j.cub.2018.05.059.
[2] X. Liu, S. Ramirez,
P.T. Pang, C.B. Puryear, A. Govindarajan, K. Deisseroth, S. Tonegawa,
Optogenetic stimulation of a hippocampal engram activates fear memory recall,
Nature. 484 (2012) 381–385. doi:10.1038/nature11028.
[3] T. Kitamura, S.K.
Ogawa, D.S. Roy, T. Okuyama, M.D. Morrissey, L.M. Smith, R.L. Redondo, S.
Tonegawa, Engrams and circuits crucial for systems consolidation of a memory,
Science. 356 (2017) 73–78. doi:10.1126/science.aam6808.
[4] D.S. Roy, S.
Muralidhar, L.M. Smith, S. Tonegawa, Silent memory engrams as the basis for
retrograde amnesia, Proc. Natl. Acad. Sci. U.S.A. 114 (2017) E9972–E9979.
doi:10.1073/pnas.1714248114.
[5] T.J. Ryan, D.S. Roy,
M. Pignatelli, A. Arons, S. Tonegawa, Memory. Engram cells retain memory under
retrograde amnesia, Science. 348 (2015) 1007–1013. doi:10.1126/science.aaa5542.
[6] D.S. Roy, A. Arons,
T.I. Mitchell, M. Pignatelli, T.J. Ryan, S. Tonegawa, Memory retrieval by
activating engram cells in mouse models of early Alzheimer’s disease, Nature.
531 (2016) 508–512. doi:10.1038/nature17172.
[7] S. Ramirez, X. Liu,
P.-A. Lin, J. Suh, M. Pignatelli, R.L. Redondo, T.J. Ryan, S. Tonegawa,
Creating a false memory in the hippocampus, Science. 341 (2013) 387–391.
doi:10.1126/science.1239073.



